Cardiac arrhythmias and covid-19: the acovid-study
The novel ACOVID-study examined a relationship between COVID-19 and arrhythmias at 6 hospitals in the Greater Copenhagen area. Between 27.04.2020 and 30.07.2020, 117 patients with laboratory-confirmed SARS-CoV-2 infections were screened for arrhythmias using the Cortrium C3⁺ Holter Monitor. The aim of the study was to estimate the type of arrhythmias with continuous electrocardiogram (ECG) in patients hospitalized with COVID-19.
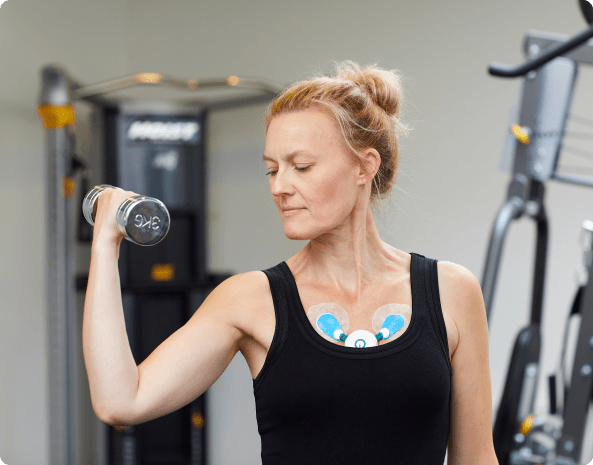
Out of a final population of 54 hospitalized patients, 15 patients developed major arrhythmias (28%). They consisted mostly of supraventricular tachycardia (22%) and new-onset atrial fibrillation/atrial flutter (4%).
COVID-19 patients were examined using the new, cablefree C3⁺ long-term ECG Holter Monitor from the Danish company Cortrium. The Cortrium C3⁺ is easy to attach to the patient with only three adhesive electrodes. The automated analysis of ECG data saves time for medical staff and gives a unique possibility to deliver scientific evidence without delay during. The results of the study will help guide physicians and authorities in the treatment of patients with COVID-19.
The ACOVID study itself has been financially supported by Innovation Fund Denmark (www.innovationsfonden.dk).
About the study
To investigate if cardiac arrhythmias is an overlooked complication of COVID-19, Ass. prof. Dr. Morten Kjøbek Lamberts from Herlev-Gentofte Hospital (Copenhagen) in partnership with Dr. Erik S. Poulsen from Cortrium ApS (Copenhagen) conducted the study over three months in spring of 2020. The “ACOVID” study is intended to provide researchers and clinicians with explanations as to whether coronavirus has an effect on heart activity and if so, which effects.
Application of the novel C3⁺ holter monitor
The Cortrium C3⁺ Holter Monitor has been used for monitoring the patients that have been included in the study. The novel Holter Monitor does not require any wires and can be attached with standard electrodes. For the study, Ambu Blue Electrodes have been used.
The monitor was placed upon day of admission or as earliest as possible during hospital stay. It remained on until discharge, transfer to the ICU or death.
The C3⁺ is very well suited for applications with a flexible duration. Recordings can be done with a length of up to seven days and are exported to the standardised european data format (.edf), with a few simple clicks in Cortrium’s software, Cortrium Apex. Throughout other applications, Cortrium Apex also supports automated, AI supported analysis options.
Background
First reports from the initial epicenter of the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China included case-series of rapid clinical deterioration of seemingly healthy individuals. Based on a cohort of 138 Chinese patients, 16.7% of patients with COVID-19 suffered from unspecified arrhythmias despite cardiac biomarkers being within normal range. In patients admitted to the ICU, arrythmias was reported in 44.4% of the patients. But, how diagnosis of arrythmias were made, was not clearly specified. Regardless of pathophysiological pathways for deterioration, of which proposed mechanisms include myocarditis, depressed cardiac function, worsening of prior cardiovascular disease or cytokine storm syndrome, one phenotypic presentation may be sudden death and arrhythmias (1).
Partners
Herlev-Gentofte University Hospital (Copenhagen)
Herlev-Gentofte Hospital houses the Copenhagen Cardiovascular Research Center focusing on nationwide register studies, large-scale clinical trials both independent and in conjunction with industry, and innovative research with emerging new technologies.
Associate Professor Morten Lamberts, MD, PhD, Principal Investigator on the ACOVID study
Morten Lamberts has a strong background within areas of pharmacoepidemiology and has increasingly focused on clinical implementable technologies. Morten Lamberts is newly appointed Innovation Ambassador at Department of Clinical Medicine, University of Copenhagen.
A position that includes facilitating clinical ideas for implementation to the next stage whether it would be proof-of-concept guidance or need for support and financing opportunities. Also, bridging the gap between the academical and clinical world with other shareholders within technical universities, private companies and large foundations is a key aspect.
For questions regarding the study please contact Associate professor Morten Lamberts [[email protected], +45 38681169]
Ambu A/S
Ambu has been bringing the solutions of the future to life since 1937. Today, millions of patients and healthcare professionals worldwide depend on the efficiency, safety and performance of our single-use endoscopy, anaesthesia, and patient monitoring & diagnostics solutions.
The manifestations of our efforts have ranged from early innovations like the Ambu® Bag™ resuscitator and the Ambu® BlueSensor™ electrodes to our newest landmark solutions like the Ambu® aScope™ – the world’s first single-use flexible endoscope. Moreover, we continuously look to the future with a commitment to deliver innovative quality products that have a positive impact on the work of healthcare professionals. Headquartered near Copenhagen in Denmark, Ambu employs approximately 3,500 people in Europe, North America and the Asia Pacific. For more information, please visit ambu.com or Ambu on LinkedIn
Innovation Fund Denmark
The Innovation Fund Denmark creates a good framework for entrepreneurs, researchers and companies in Denmark to develop innovative and viable solutions to the concrete challenges facing society. All projects are subject to requirements in terms of commitment, transparency, education and community ethics. More about Innovation Fund Denmark can be found at www.innovationsfonden.dk
(1) Morten Lamberts, MD, PhD; Bochra Zareini, MD, PhD; Deepthi Raja, BMSc; Mohammed El-Sheikh, BMSc; Morten Schou, MD, PhD; Gunnar Gislason, MD, PhD: Cardiac arrhythmias in patients hospitalized with COVID-19: The ACOVID-study; Published March 22, 2021, DOI: https://doi.org/10.1016/j.hroo.2021.03.008
